45 label the ph scale
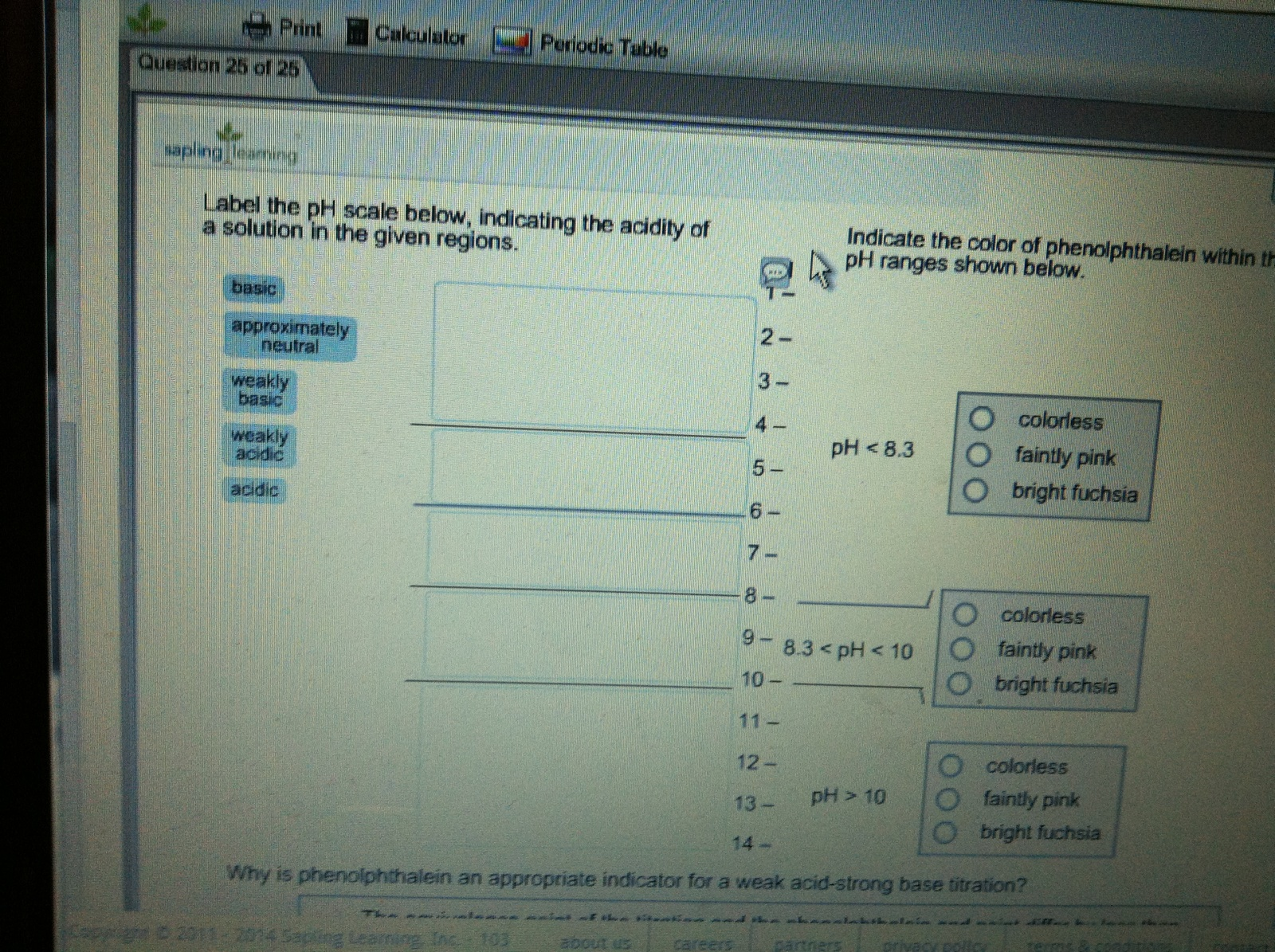
Solved LAbel the pH scale below, indicating the acidity of a | Chegg.com Indicate the color of phenoiphthalein within the pH ranges shown belovw pH O colorless |° pH< 8.3 faintly pink bright fuchsia O neutra O colorless 98.3 How to Read pH Strips: 9 Steps (with Pictures) - wikiHow 3. Dip one end of the test strip in the substance you want to test. You don't need to submerge the whole strip in the test substance. Hold the strip at one end and dip the other end in the liquid, then remove it after the appropriate length of time. [3] You can use pH strips to test the pH level of any liquid. 4.
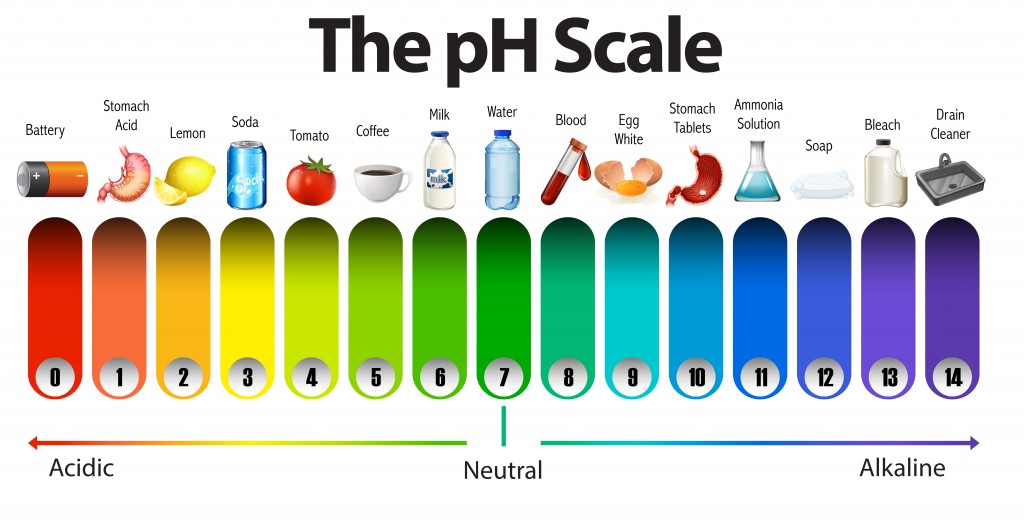
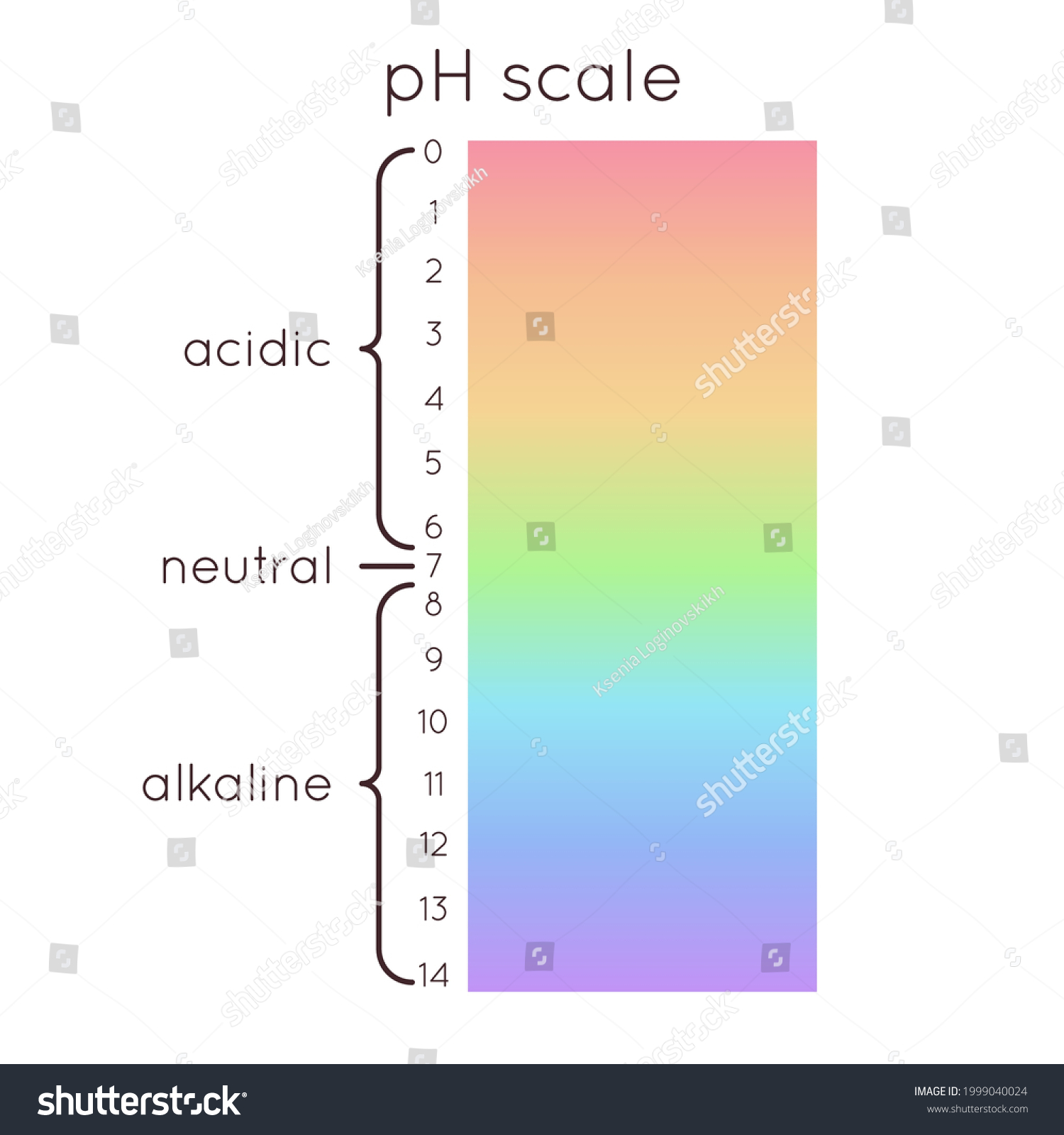
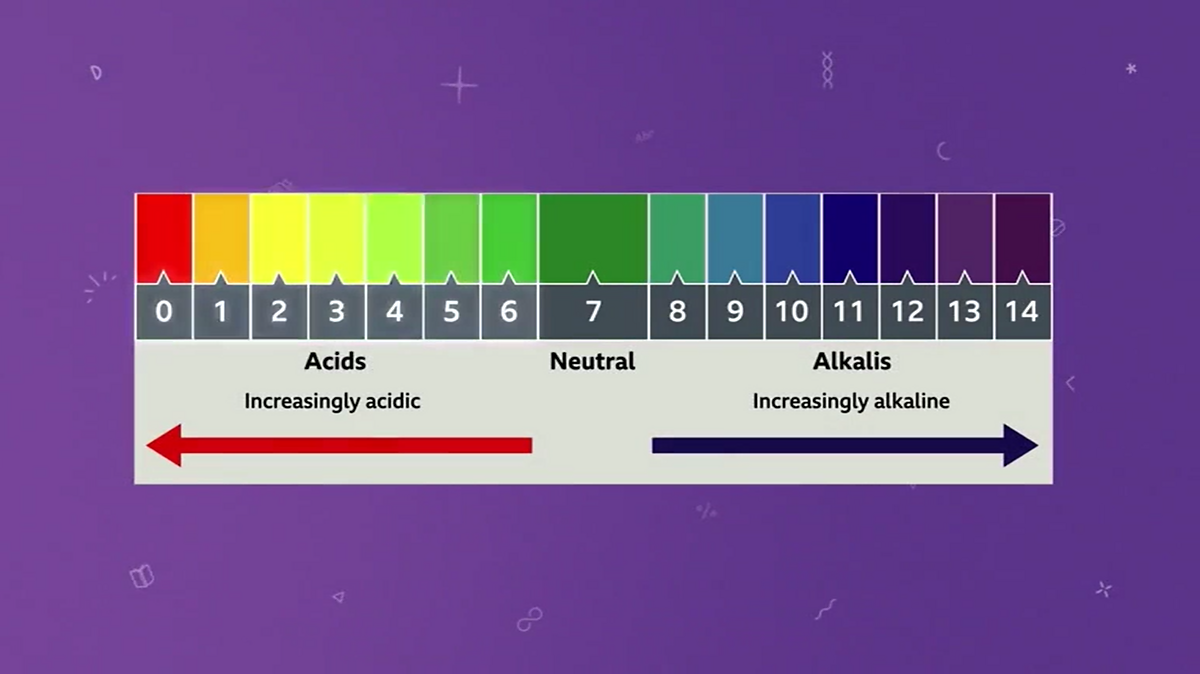
The pH Scale | Biology for Majors I | | Course Hero The pH scale is, as previously mentioned, an inverse logarithm and ranges from 0 to 14 (Figure 1). Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Extremes in pH in either direction from 7.0 are usually considered inhospitable to life.
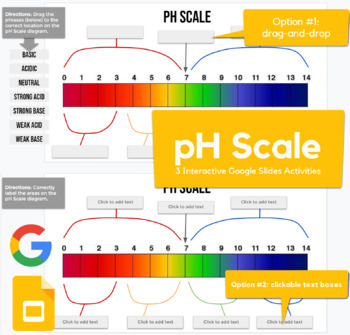
Label the ph scale
Draw neat and labeled diagram of pH scale? - Toppr Ask The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 The pH Scale - GitHub Pages This is known as the pH scale The range of values from 0 to 14 that describes the acidity or basicity of a ... Example 12. Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5; pure water, pH = 7; wine, pH = 3.0; Solution. With a pH greater than 7, milk of magnesia is basic. (Milk of magnesia ... pH Scale Flashcards | Quizlet pH. abbreviation meaning "potential for hydrogen." pH Scale. a scale used to define the levels of hydrogen (H) or hydroxide (OH) ions in a solution. Ranges from 0 (acidic) to 14 (basic/alkaline), with 7 being neutral. Hydrogen Ion. an ion of hydrogen (H) created by the breaking up of a water molecule during the mixing of a solution.
Label the ph scale. The pH Scale - Science Buddies In order to deal with these large numbers more easily, scientists use a logarithmic scale, the pH scale. Each one-unit change in the pH scale corresponds to a ten-fold change in hydrogen ion concentration. The pH scale is theoretically open-ended but most pH values are in the range from 0 to 14. Labeling the pH scale Quiz - PurposeGames.com About this Quiz. This is an online quiz called Labeling the pH scale. There is a printable worksheet available for download here so you can take the quiz with pen and paper. Total Points. 0. Get started! Today's Rank. --. 0. Label The Ph Scale Diagram / Science Worksheets Label Color The Ph ... With specific ph values, then draw and label those substances in the worksheet. The scale ranges from 0 to 14. The ph scale measures how acidic or basic a substance is. Ph 7 corresponds to neutral ph. Ph less than 7 corresponds to acidic . Acidity and basicity, proton concentration, the ph scale, and buffers. The pH Scale - Introductory Chemistry - 1st Canadian Edition Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5 pure water, pH = 7 wine, pH = 3.0 Solution With a pH greater than 7, milk of magnesia is basic. (Milk of magnesia is largely Mg (OH) 2 .) Pure water, with a pH of 7, is neutral. With a pH of less than 7, wine is acidic. Test Yourself
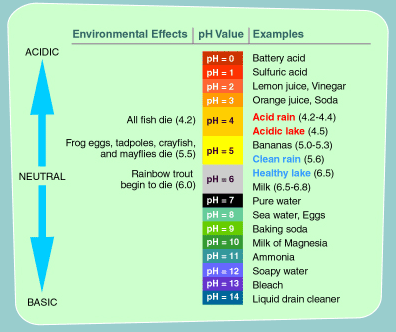
pH Scale - Acids and Bases A pH of 7 is neutral on the scale, greater than 7 is a base and less than 7 is an acid. Strong acids are mostly ranged at a pH of 0-2, strong bases have a range at a pH of 12-14. Colour Indicators. Colour Indicators are used to determine how acidic, basic or neutral the solution is. This method is told by the changing colour of the substance ... How do you draw the pH scale? - Answers The pH scale is often indicated as a vertical bar graph with scaled numbers from 0 to 14 (top to bottom). The lower numbers at the top are the more acidic pH, while the higher numbers near the ... pH Scale | U.S. Geological Survey - USGS.gov The pH scale measures how acidic an object is. Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. The pH scale - Acids, bases and salts - (CCEA) - BBC Bitesize The pH scale measures a solution's acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali). pH 0 - 2: strong acid pH 3 - 6: weak acid pH 7: neutral pH 8 ...
15.4: The pH Scale - Chemistry LibreTexts The constant of water determines the range of the pH scale. To understand what the pK w is, it is important to understand first what the "p" means in pOH, and pH. The Danish biochemist Søren Sørenson proposed the term pH to refer to the "potential of hydrogen ion." ... [pK_w= pH + pOH = 14 \label{5b} \] Definitions. A solution with more \(OH ... pH Chemistry (Acids & Bases) - Definition, Calculating pH Value, Videos ... pH Chemistry. A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14: Litmus paper is an indicator used to tell if a substance is an acid or a base. The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. For example, Vinegar is an acid and measures 2.4 on ... PDF pH Scale Activity - birdvilleschools.net pH Scale Activity 1. On the construction paper, NEATLY draw a pH scale. 2. Scale the line from 0 to 14 with a mark for each number. 3. Cut out words & paste the labels in correct areas of pH scale. Weak Acid Strong Acid Strong Base Weak Base Neutral . 4. Color & Label the pH on the picture. Cut out & paste in the correct sections of the pH scale. Label The Ph Scale / Draw A Ph Scale And Label Water Hydrochloric Acid ... Start studying label the ph scale. With specific ph values, then draw and label those substances in the worksheet. Label each solution as acidic, basic, or neutral based only on the stated ph. Michael heim / eyeem / getty images at 25 c, the ph of pure water is very close to 7. Naoh Ph Level At Level from i2.wp.comFree answer to thanks in advance!
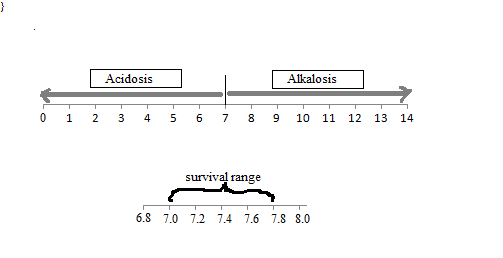
Acids, Alkalis, and the pH Scale - Compound Interest The pH of the stomach can vary, between 1.5 and 3.5 on the pH scale. However, this has no effect on the pH of our body, or, more specifically, our blood. Human blood has a pH value that's always slightly alkaline, between 7.35-7.45.
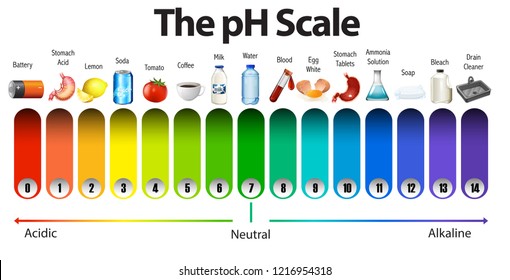
The pH scale with some common examples The pH scale, with examples of common solutions and their pH values. Download/View. For commercial use please contact us.
pH Scale | U.S. Geological Survey - USGS.gov pH is a measure of how acidic/basic water is. The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens.
Solved Label the pH scale below, indicating the acidity of a | Chegg.com Label the pH scale below, indicating the acidity of a solution in the given regions. Indicate the color of phenolphthalein within the pH ranges shown below. Why is phenolphthalein an appropriate indicator for a weak acid-strong base titration? A.
The pH scale - BBC Bitesize The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify as acidic, alkaline or neutral. Neutral solutions are...
The pH Scale | Biology for Non-Majors I | | Course Hero The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Most parts of our body (excluding things like stomach acid) measure around 7.2 and 7.6 on the pH scale (a 7 is neutral on the scale). If foreign strong substances dramatically change this pH, our bodies can no longer function properly.
What is the pH Scale? - ReAgent Chemical Services The pH scale measures the relative acidity and alkalinity of solutions. It's a negative logarithmic scale of base ten that measures the potential of a solution to accept protons in the form of hydrogen ions. For example, a solution with pH level 8 is ten times more alkaline than pure water, which has a pH level of 7.
pH Scale Flashcards | Quizlet pH. abbreviation meaning "potential for hydrogen." pH Scale. a scale used to define the levels of hydrogen (H) or hydroxide (OH) ions in a solution. Ranges from 0 (acidic) to 14 (basic/alkaline), with 7 being neutral. Hydrogen Ion. an ion of hydrogen (H) created by the breaking up of a water molecule during the mixing of a solution.
The pH Scale - GitHub Pages This is known as the pH scale The range of values from 0 to 14 that describes the acidity or basicity of a ... Example 12. Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5; pure water, pH = 7; wine, pH = 3.0; Solution. With a pH greater than 7, milk of magnesia is basic. (Milk of magnesia ...
Draw neat and labeled diagram of pH scale? - Toppr Ask The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0
























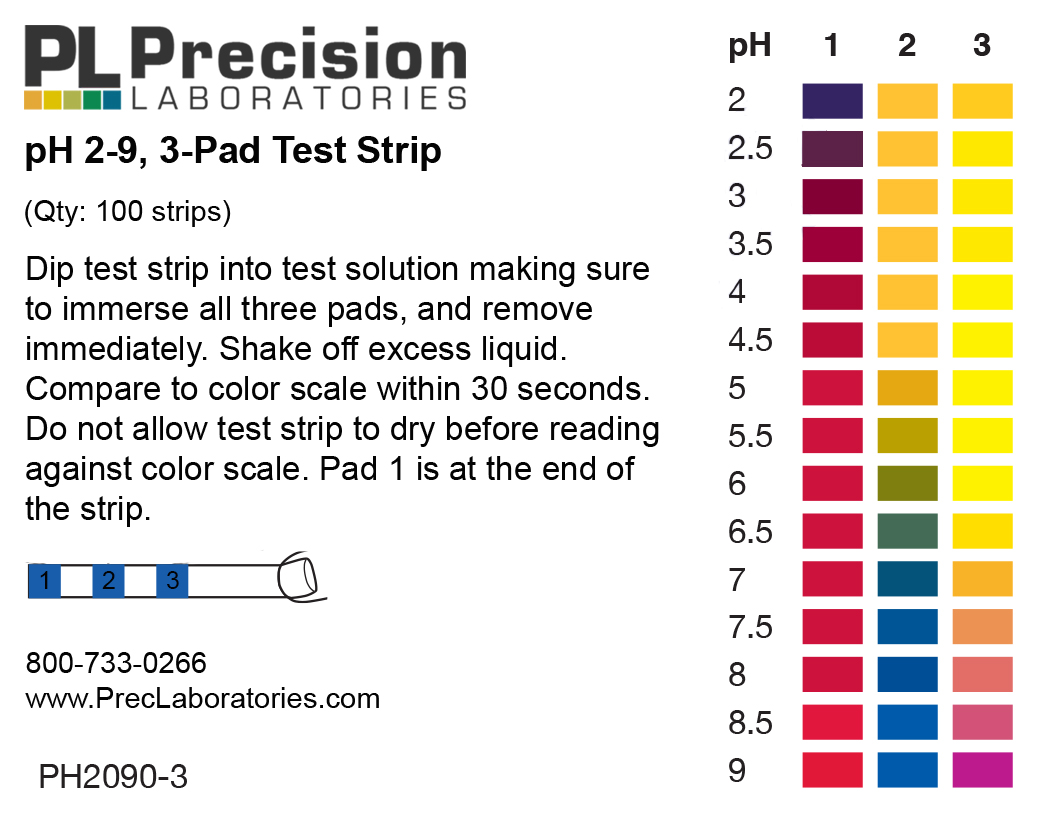

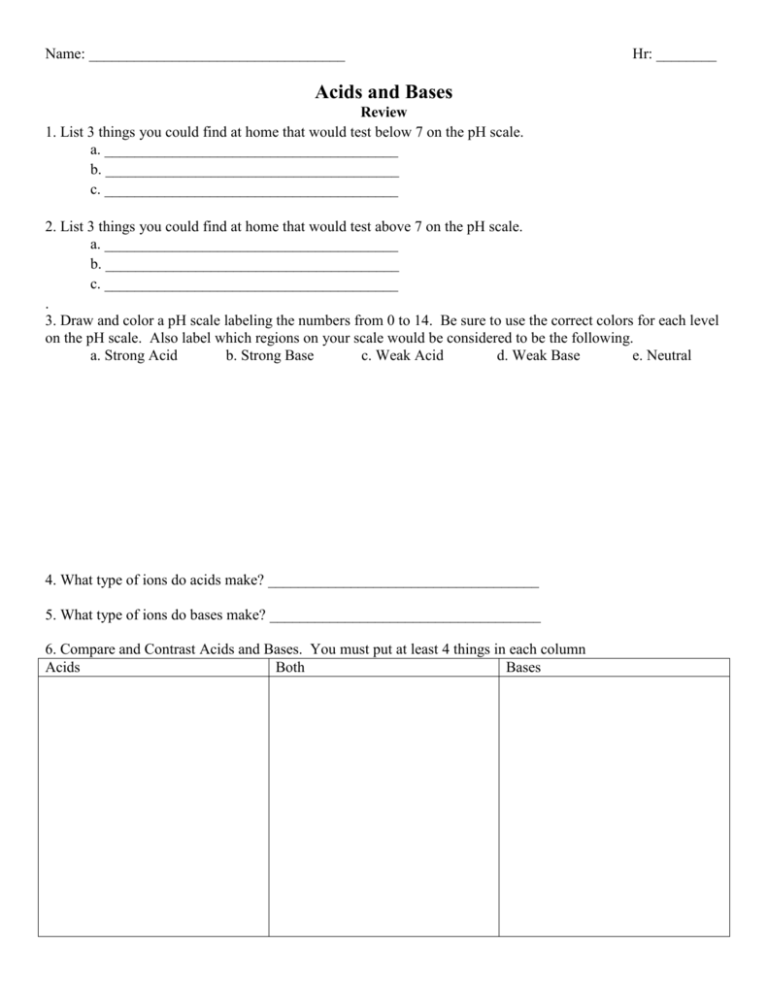
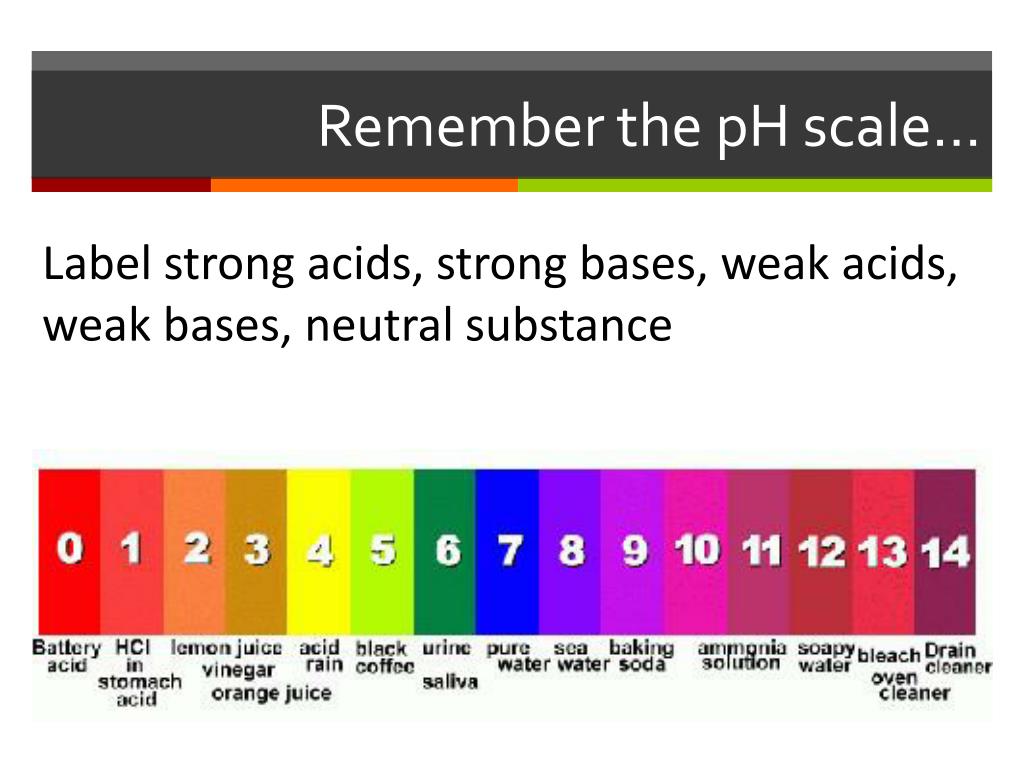
Post a Comment for "45 label the ph scale"