45 open label clinical trial
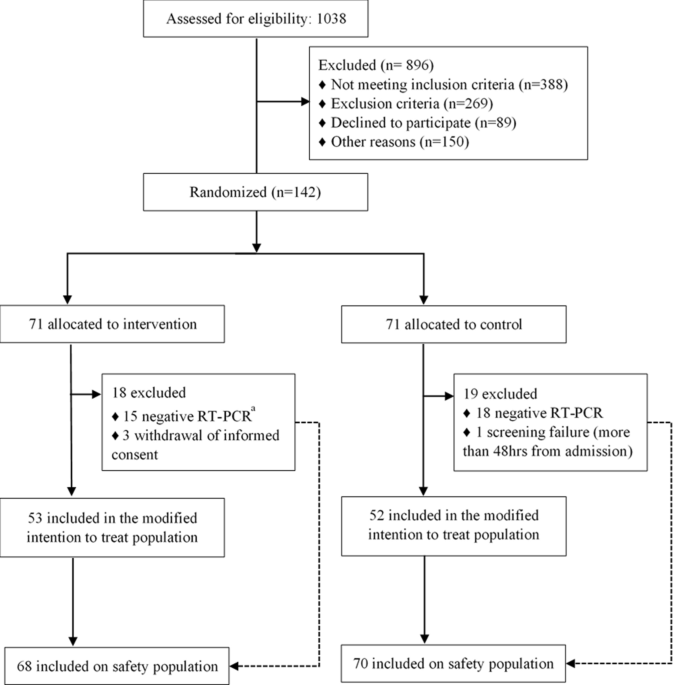
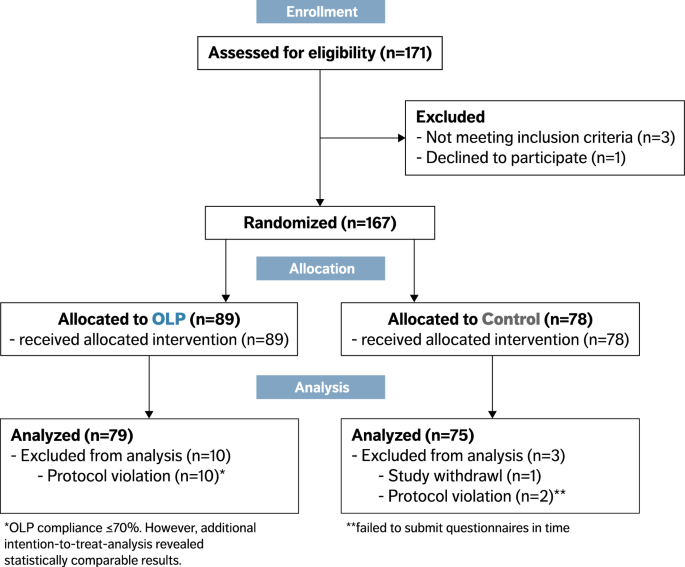
An open label randomized clinical trial of Indomethacin for mild and ... Study design. This open-label randomized clinical trial consisted of two parallel groups; the control group with paracetamol and standard of care (SOC) and the indomethacin group with SOC. Dexamethasone versus methylprednisolone for critical asthma: A single ... The Ideal STeroids for Asthma Treatment in the PICU (iSTAT PICU) trial ( , NCT03900624) was an investigator-initiated, single center, open-label, non-randomized, two-arm, parallel-group trial conducted at Johns Hopkins All Children's Hospital from April 2019 through December 2021. In response to the COVID-19 pandemic ...
Get the Scoop on an Open-Label Clinical Trial [2023 Guide] An open-label clinical trial is a type of clinical trial in which the volunteers have access to the information about the treatment they are being given. Open-label clinical trials are the opposite of blinded experiments. In blinded experiments, the information is withheld from the participants to avoid bias.
Open label clinical trial
Effects of open-label placebos in clinical trials: a ... - Nature Abstract Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and... Open-label Clinical Trial of Niraparib Combined With ... - JAMA Design, Setting, and Participants This open-label, single-arm, phase 2 study enrolled 55 eligible patients with advanced or metastatic TNBC irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression at 34 US sites. Data were collected from January 3, 2017, through October 29, 2018, and analyzed from October 29, 2018 ... en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the ...
Open label clinical trial. › understanding-clinicalUnderstanding Clinical Trial Terminology: What is an Open ... Jun 24, 2019 · Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ... Phase 3, Open-label Clinical Trial of EB-101 for the Treatment of ... This open-label, controlled study will evaluate the efficacy and safety of EB-101 for the treatment of large, chronic, RDEB wounds. The study intervention consists of one-time surgical application of gene-corrected keratinocyte sheets (EB-101) for the treatment of RDEB wound sites in up to approximately 10-15 participants. › life-sciences › What-is-anWhat is an Open-Label Clinical Trial? - News-Medical.net Mar 31, 2022 · Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play... › guides › what-is-an-open-labelWhat is an Open Label Study in Clinical Research? | Power In an open label study, or open label trial, all participants know exactly what treatment/intervention they will be receiving in the study. Study physicians and investigators also know what each participant is receiving. That means that there is no uncertainty regarding whether or not you may be receiving a placebo rather than the study treatment.
Spotlight on Open-Label Extension Studies The stated objective of most OLE studies is to obtain long-term safety and tolerability data. Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study. Quoin Pharmaceuticals Doses First Patient in Open Label Netherton ... ASHBURN, Va., March 21, 2023 (GLOBE NEWSWIRE) — Quoin Pharmaceuticals Ltd. (NASDAQ: QNRX) (the "Company" or "Quoin"), a clinical stage, specialty pharmaceutical company focused on rare and orphan diseases, today announces the dosing of the first patient in its open label clinical trial in Netherton Syndrome patients.The trial is a single arm, open label study, investigating the ... Quoin Pharmaceuticals Doses First Patient in Open Label Netherton ... The trial is a single arm, open label study, investigating the safety and efficacy of Quoin's lead candidate, QRX003, in Netherton Syndrome patients who are currently receiving off-label ... VILTEPSO® (viltolarsen) injection Open Label Extension Clinical Trial ... NS Pharma is pleased to announce participation in the Muscular Dystrophy Association's Clinical & Scientific Congress 2023 being held in Dallas, Texas. The company will be presenting previously reported long-term efficacy and safety data from the open-label extension of a Phase 2 study of VILTEPSO®.
Open-Label Trial | NIH - HIV.gov Open-Label Trial A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term (s) Clinical Trial Double-Blind Study Reducing bias in open-label trials where blinded outcome ... - PubMed Results: TRIGGER was an open-label cluster randomised trial whose primary outcome was further bleeding. Because of the cluster randomisation, all researchers in a hospital were aware of treatment allocation and so could not perform a blinded assessment. Phase 3 Open-Label Clinical Trial of Elexacaftor/Tezacaftor/Ivacaftor ... Methods: In this Phase 3, open-label, two-part study (Parts A and B), children weighing <14 kg (on Day 1) received elexacaftor 80 mg once daily, tezacaftor 40 mg once daily, and ivacaftor 60 mg each morning and 59.5 mg each evening; children weighing ≥14 kg received elexacaftor 100 mg once daily, tezacaftor 50 mg once daily, and ivacaftor 75 ... Open-label placebo clinical trials: is it the rationale, the ... Clinical trials into open-label placebos (OPL) appear to show promise for a range of self-reported conditions but have been hampered by small sample sizes and short duration. What are the new findings? Placebo concepts refer to: (1) 'methodological controls'; or (2) 'mind-body treatment interventions'. ...
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... Clinical trials are important research studies that are conducted to test potential new medicines in people after earlier stages of research have been successfully completed. Clinical trials typically move forward in a series from early-stage, small-scale Phase 1 studies to late-stage, large-scale, Phase 3 studies. These studies could be double-blind in which case the trial […]
› news-releases › viltepso-viltoVILTEPSO® (viltolarsen) injection Open Label Extension ... Mar 19, 2023 · Data were from a final four-year analysis (up to Week 216) of the open-label extension trial of a VILTEPSO Phase 2 study. NS Pharma, Inc. (NS Pharma; President, Tsugio Tanaka), is a wholly owned ...
A phase I open-label clinical trial to study drug-drug interactions of ... This is a phase 1, open-label, single-sequence, multiple-dose, single-center trial conducted in the US (NCT03790839), to evaluate the clinical pharmacokinetics, safety and pharmacodynamics of dorzagliatin co-administered with sitagliptin in patients with T2D and obesity.The trial has completed. 15 patients with T2D and obesity were recruited and treated with sitagliptin 100 mg QD on Day 1-5 ...
Open-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 An open-label trial of escitalopram for SAD patients (N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of …
› article › releasesQuoin Pharmaceuticals Doses First Patient in Open Label ... 2 days ago · ASHBURN, Va., March 21, 2023 (GLOBE NEWSWIRE) -- Quoin Pharmaceuticals Ltd. (NASDAQ: QNRX) (the “Company” or “Quoin”), a clinical stage, specialty pharmaceutical company focused on rare and orphan diseases, today announces the dosing of the first patient in its open label clinical trial in Netherton Syndrome patients.
Open-label extension studies: do they provide meaningful ... - PubMed Most of the value accrued in open-label extension studies is gained from a refinement in the perception of the expected incidence of adverse effects that have most likely already been identified as part of the preclinical and clinical trial programme.
Guidance for Clinical Trial Sponsors - Food and Drug Administration Guidance for Clinical Trial Sponsors . Establishment and Operation of Clinical Trial Data Monitoring Committees . For questions on the content of this guidance, contact the Office of Communication ...
FDA Guidance: "Design Considerations for Pivotal Clinical ... l A one-arm study is an observational study—not a clinical trial or an experiment. ... - For non-masked (open label) studies this means before any patient reaches an outcome. 36.
ADP-A2M4CD8 in HLA-A2+ Subjects With MAGE-A4 Positive Esophageal or ... This study will investigate the efficacy of ADP-A2M4CD8 T-cell therapy in subjects who have the appropriate human leukocyte antigen (HLA) and tumor antigen status and whose esophageal or esophagogastric junction (EGJ) cancer expresses the MAGE-A4 protein. Study Design Go to Resource links provided by the National Library of Medicine
What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities.
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... End of Trial and Open-Label Extension (OLE) Frequently Asked Questions (FAQ) When did the solanezumab ("sola") and gantenerumab ("gant") blinded drug arms in the DIAN-TU trial officially end? When did the DIAN-TU announce the results of the current trial drug arms of solanezumab and gantenerumab; and where can I look for the results?
finance.yahoo.com › news › quoin-pharmaceuticalsQuoin Pharmaceuticals Doses First Patient in Open Label ... Mar 21, 2023 · The trial is a single arm, open label study, investigating the safety and efficacy of Quoin’s lead candidate, QRX003, in Netherton Syndrome patients who are currently receiving off-label ...
en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the ...
Open-label Clinical Trial of Niraparib Combined With ... - JAMA Design, Setting, and Participants This open-label, single-arm, phase 2 study enrolled 55 eligible patients with advanced or metastatic TNBC irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression at 34 US sites. Data were collected from January 3, 2017, through October 29, 2018, and analyzed from October 29, 2018 ...
Effects of open-label placebos in clinical trials: a ... - Nature Abstract Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and...



















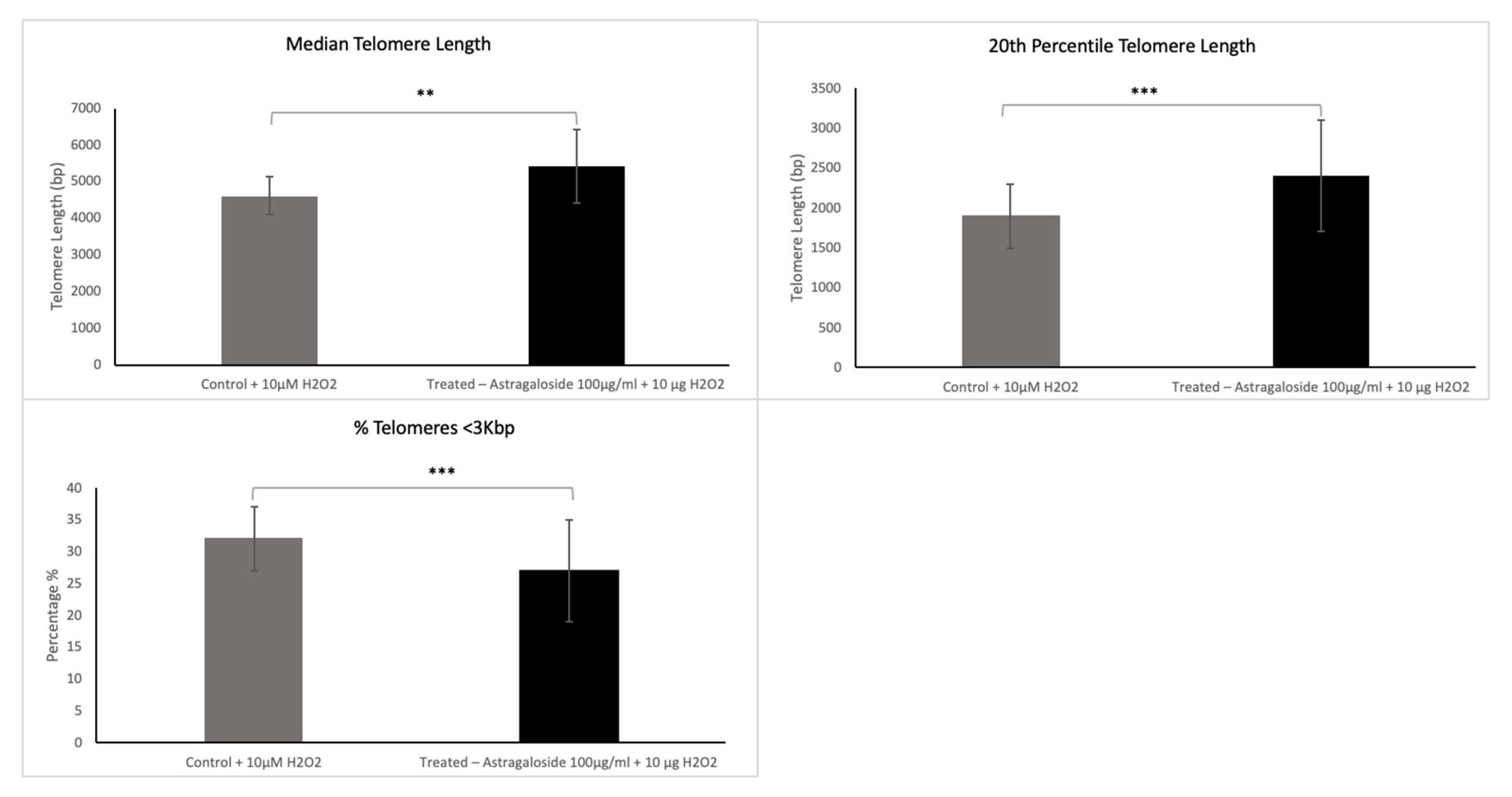





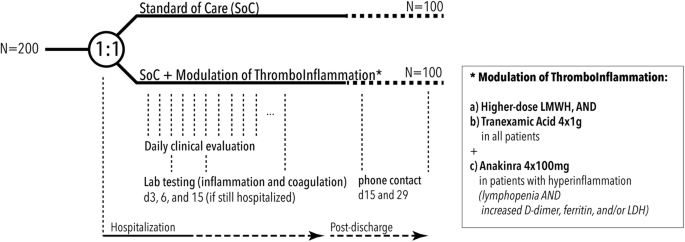
30704-x.fp.png)
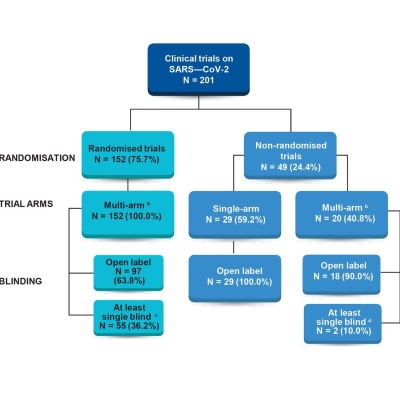




Post a Comment for "45 open label clinical trial"